New Infusion Therapies for Parkinson’s Disease
There are two newly FDA-approved treatments available for people with Parkinson’s disease (PwP) who experience motor fluctuations—periods of varying symptom control throughout the day. These fluctuations can include motor symptoms such as tremor, stiffness, slowness, and dyskinesias, which are the involuntary, wiggly movements that can occur as a side effect of medication.
Instead of taking oral medications several times a day, these infusions allow for a small dose of medication to be delivered continuously under the skin. You can think of them as being similar to the pumps used by some people with diabetes to control their blood sugar.
Many people with Parkinson’s disease (PD) have problems in the gastrointestinal tract—such as irregular or slowed stomach emptying or constipation—which can impact medication absorption and effectiveness. An under-the-skin infusion may therefore provide better benefits and more predictable symptom control.
There are currently two infusion therapies: one with carbidopa/levodopa and the other with apomorphine, a dopamine agonist. Dopamine agonists mimic dopamine, the brain chemical that affects movement and decreases in people with Parkinson’s, whereas levodopa is converted into dopamine by neurons in the brain.
The apomorphine pump has been approved in Europe for a long time but was only available in the U.S. as a rescue injection until this year. Previously, people would inject a dose themselves when they experienced an “off” period—a time when their Parkinson’s motor symptoms were not well controlled.
The Two New Infusion Therapies
Vyalev
Vyalev is a 24-hour infusion of carbidopa/levodopa. It is administered as “foscarbidopa/foslevodopa.” The “fos” part indicates that this is a prodrug, meaning it is converted into carbidopa/levodopa once inside the body. This prodrug form allows for more controlled medication delivery.
The continuous delivery through the pump provides more stable dopamine levels in the brain compared to traditional oral medications. The pump must be worn in a sling or fanny pack but can be disconnected for up to three hours at a time (e.g., for swimming or showering). It also allows for small additional boosts or loading doses when extra medication is needed.
Currently, Vyalev is only covered for people with Parkinson’s who are under 65 years of age and who experience at least 2.5 hours per day of inadequate symptom control. However, insurance coverage is expected to expand to include Medicare and Medicaid sometime in 2025.
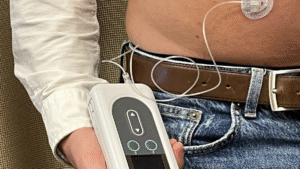
Vyalev Pump
Onapgo
Onapgo is the newly approved apomorphine pump, which received FDA approval in January 2025 and is expected to hit the market in the second quarter of 2025. Despite its name, apomorphine does not contain morphine or any narcotics. Like Vyalev, this pump must be worn in a fanny pack or sling but can be disconnected when needed.
The goal is to provide consistent dosing to improve symptom control, particularly for people with progressing Parkinson’s who may not achieve adequate symptom relief with pills or who experience motor fluctuations. The exact insurance coverage details for Onapgo remain unknown at this time.
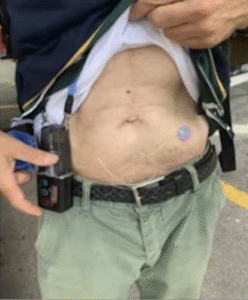
Onapgo Pump
Common Considerations for Both Pumps
Since both Onapgo and Vyalev are subcutaneous infusions (delivered under the skin), they can cause skin-related side effects, including:
- Redness, pain, bruising, infection, and skin nodules at the infusion site
- Lightheadedness
- Worsening dyskinesias (involuntary movements)
- Hallucinations
It is essential to discuss all potential side effects with your medical provider to determine whether these therapies are right for you.
While these medications themselves are not new, the approval of these infusion therapies is an exciting advancement. More treatment options are always welcome news. As Parkinson’s symptoms progress, these pumps have the potential to significantly improve the lives of many people with PD and their families. Since there is no one-size-fits-all approach to Parkinson’s, having new options to personalize care is a major step forward!